Context:
Recently, Union Health ministry proposed a separate post for the medical devices controller.
About the News
 News Source: BS
News Source: BS
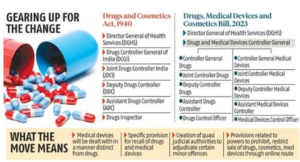
- The Union Health Ministry has presented a draft of the New Drugs, Medical Devices and Cosmetics Bill, 2023, which seeks to replace the Drugs and Cosmetics Act of 1940.
Central Drugs Standard Control Organisation (CDSCO)
|
- Draft proposed the creation of a separate post for a drugs and medical devices controller general in India, under whom a separate wing of officials would operate as regulators for the medical devices sector.
- As of now, medical devices are treated as drugs and there is no separate definition for those.
- Currently, manufacturing activities relating to drugs and cosmetics are regulated by only the state governments through their drug control organizations.
- Medical devices officers independent of drug control officers and creation of medical devices testing laboratories and the appointment of medical devices testing officers.
 News Source: BS
News Source: BS
Post Views: 195
